
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). Neutron and Mass Numbers and Nuclear Properties For example, the neutron number of uranium-238 is 238-92=146. We can determine the neutron number of certain isotope.

Therefore, we cannot determine the neutron number of uranium, for example. Each nuclide is denoted by chemical symbol of the element (this specifies Z) with tha atomic mass number as supescript. The various species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides. Nuclides that have the same neutron number but a different proton number are called isotones. Neutron number is rarely written explicitly in nuclide symbol notation, but appears as a subscript to the right of the element symbol. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z. Neutron number plus atomic number equals atomic mass number: N+Z=A. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Mass numbers of typical isotopes of Neon are 20 21 22. This fact has key implications for the building up of the periodic table of elements. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. In the periodic table, the elements are listed in order of increasing atomic number Z.
The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The configuration of these electrons follows from the principles of quantum mechanics. The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. See also: Atomic Number – Does it conserve in a nuclear reaction? Atomic Number and Chemical PropertiesĮvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements. In a neutral atom there are as many electrons as protons moving about nucleus.
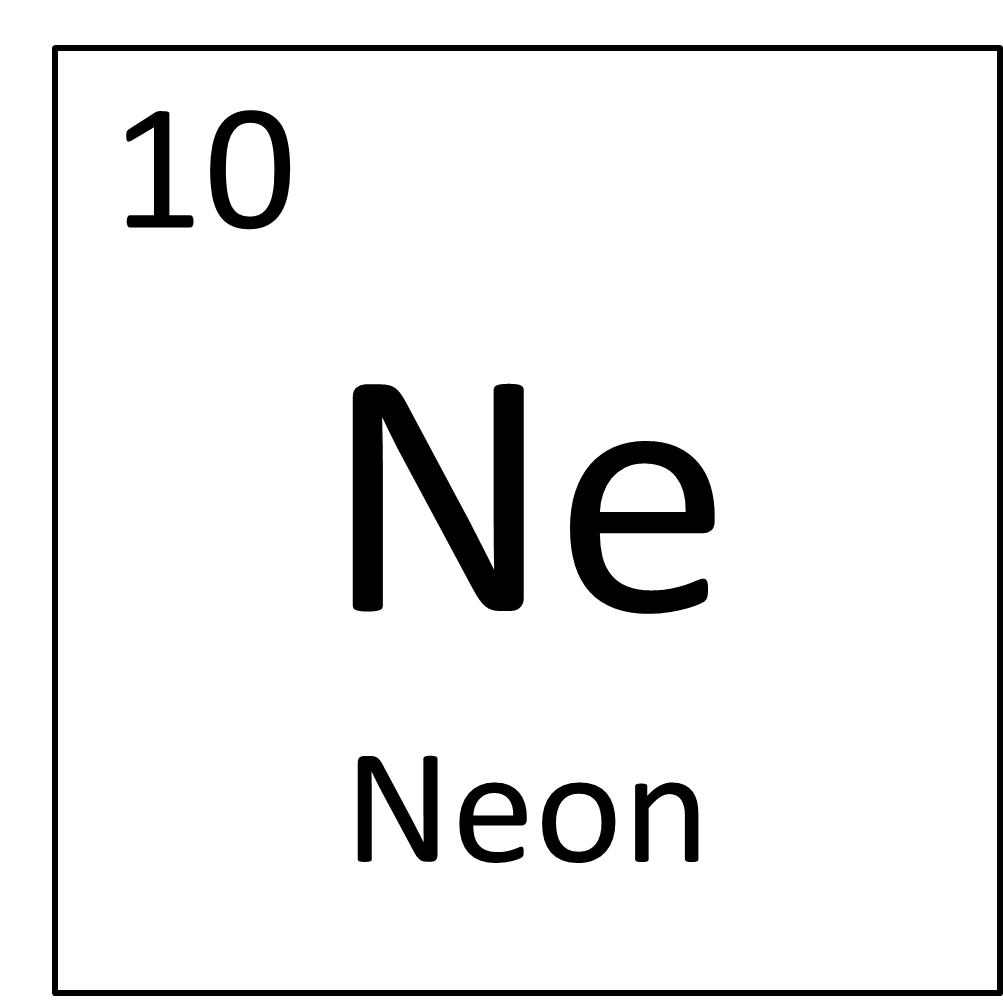
The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. K) - Thermal Conductivity 0.0493 Specific Heat 0.904 Heat of Fusion 0.3317 Heat of Vaporization 1.7326 Atomic Number of Neon.Neon – Properties Element Neon Atomic Number 10 Symbol Ne Element Category Noble Gas Phase at STP Gas Atomic Mass 20.1797 Density at STP 0.9 Electron Configuration 2s2 2p6 Possible Oxidation States 0 Electron Affinity - Electronegativity - 1st Ionization Energy 21.5645 Year of Discovery 1898 Discoverer Ramsay, William & Travers, Morris Thermal properties Melting Point -248 Boiling Point -248.7 Thermal Expansion µm/(m


 0 kommentar(er)
0 kommentar(er)
